 当前位置:首页 > 新闻动态 > 行业新闻
当前位置:首页 > 新闻动态 > 行业新闻新冠肺炎“炎症风暴”是近期公众关注的诸多热点话题之一;所谓“炎症风暴”即全身炎症反应综合征(SIRS),人体的炎症因子,不仅可以杀掉病毒,也会给自身造成损害,某些新冠患者后期可能突然启动了一个“炎症风暴”,结果导致各个器官的功能衰竭。那么应该如何应对“炎症风暴”呢?中南大学湘雅医学院林韶辉博士撰文“有效针对新冠肺炎的“炎症风暴”——Omega-3多不饱和脂肪酸”,表达了自己的一些观点,或许会给大家一些思考和启发。
一、新冠肺炎专家说
1. 武汉金银潭医院:上海中山医院重症医学科副主任钟鸣医生(2020年1月24到达武汉),2020年2月3日,南方人物周刊对钟医生进行独家专访,提到救治初期,所面临的困难之中,有的新冠患者后期可能突然启动了一个“炎症风暴”,这种炎症风暴导致了各个器官的功能衰竭,一旦进入这个状态,我们的治疗很难把它拉回来。
2. 武汉大学中南医院:《美国医学会杂志》2月7日在线发布了武汉大学中南医院重症监护室(ICU)主任彭志勇博士领衔的一项回顾性分析,研究团队认为,新冠肺炎致死的三大主要机制:
中性粒细胞增多,与细胞因子风暴有关;
D-二聚体升高反映凝血激活,提示持续的炎症反应;
血尿素升高提示急性肾损伤,这是感染、休克和缺氧的综合结果。
3. 种种迹象表明,这些重症患者中发生了不可逆转的“炎症风暴”。而一旦患者发生“炎症风暴”,糖皮质激素是对抗炎症风暴的武器之一,可以给免疫系统“灭火”,减轻机体损伤。不过,糖皮质激素是一把“双刃剑”。一方面它可以减轻炎症反应,有利于改善缺氧、呼吸窘迫症状;但长期大剂量使用也可能引发诸多不良反应。不少经过大剂量激素治疗的“非典”患者,都留下了如股骨头坏死等严重后遗症。
二、“炎症风暴”与Omega-3多不饱和脂肪酸
3. Omega-3 PUFA对抗炎症反应的作用机制
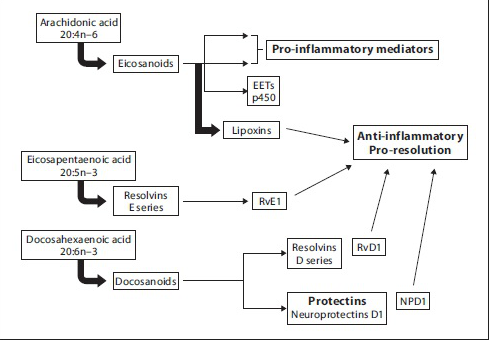
图1. 长链不饱和脂肪酸代谢示意图
4. 多种文献证明,为了减少患者还可能发生爆发性炎症反应,使用高纯度的 Omega-3 PUFA制剂,可以减低炎症风暴的发生风险。具体表现
4.1 Omega-3 PUFA富集于细胞膜,参与炎症反应调控过程,减少炎性细胞产生促炎性细胞因子;
Omega-3 PUFA富集于细胞膜,提高细胞膜EPA和DHA脂肪酸的比例[11, 12],影响胞内信号传导,抑制炎症相关的转录因子NF-kappa B的转录活性[13, 14],从而减少促炎性细胞因子TNF-alpha、IL-6 和IL-8等的表达[15]。
花生四烯酸类脂质经一系列酶催化产生促炎症调节作用的前列腺素(PGE2)和白三烯B4等。这些促炎性介质进而可激活中性粒细胞、巨噬细胞,刺激炎症细胞因子TNF-α、IL-1、IL-2、IL-8和干扰素(Interferon, IFN)等释放增多。Omega-3和Omega-6两类 PUFA竞争相同的脂肪酸代谢酶(Elovl5和FADS2)系统[16-18],从而干扰促炎性介质的生成。
EPA和DHA经代谢产生消散素(Resolvins)和保护素(Protections)。实验研究证实,消散素和保护素在关节炎[19]、结肠炎[20]、哮喘[21, 22]等多种炎性疾病中发挥重要的抗炎作用[23, 24] 。例如,resolvin E1,resolvin D1和protectin D1能抑制浸润性中性粒细胞的细胞迁移,从而减轻局部炎症[24]。此外,resolvin D1和protectin D1还能抑制TNF-α and IL-1β的产生,从而减弱炎症反应[25, 26]。
5. Omega-3 PUFA缓解炎症的具体临床获益
5.1 C反应蛋白等炎症指标显著下降,血红蛋白和白细胞、血小板等细胞类群总量显著上升。
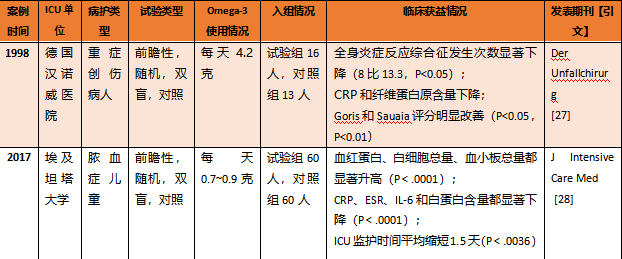
表1. ICU患者使用Omega-3 PUFA带来的生理指标和临床指标获益
5.2 败血症状明显下降,治疗用抗生素使用时间缩短,全身炎症反应综合征减少,器官功能障碍后遗症发生率减少,死亡率下降;
美国田纳西大学附属医院和西班牙多所医院的临床研究表明,对于重症创伤患者和脓毒症患者等ICU患者,每天1~3克Omega-3 PUFA营养液可以有效降低菌血症、败血症并发症发生(下降14%[29]~35%[30]),明显降低治疗用抗生素使用时间和腹腔内脓肿发生率[30]。而使用更高Omega-3 PUFA含量(4克以上)的营养液后,创伤病人全身炎症反应综合征发生人数显著下降[27];脓毒症、败血症患者的器官功能障碍发生率减少达到43%[31],死亡率显著下降13.1%[29]~19.4%[31]。
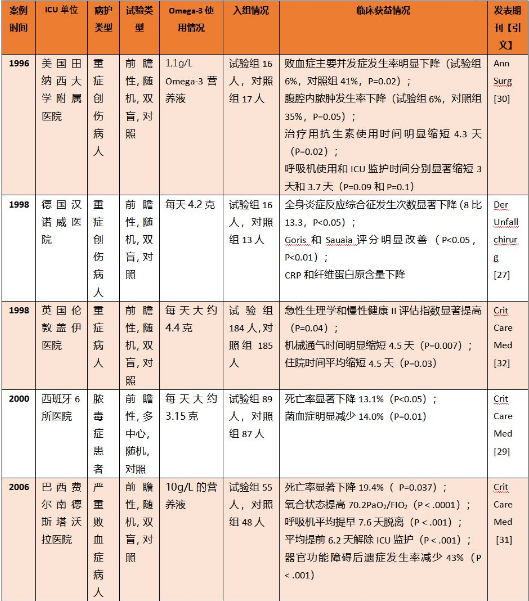
表2. 创伤患者和败血症患者使用Omega-3 PUFA的临床获益
5.3 改善氧合状态,减少呼吸机使用,缩短ICU监护时间;
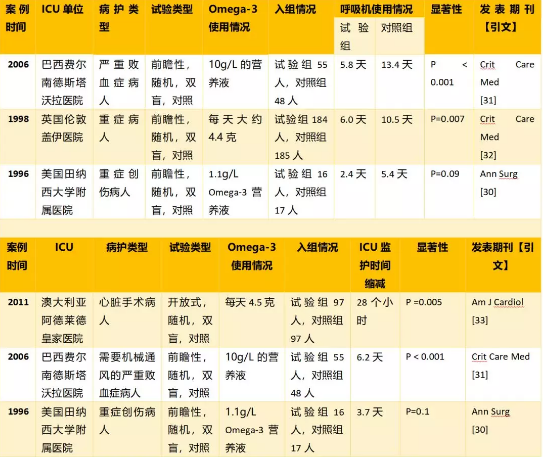
6.2 生物利用度高,保证体内有效富集。
6.3 添加中链甘油三酯(MCTs),促进Omega-3 PUFA快速细胞富集,为重症患者的症状缓解赢得时间。添加MCT的Omega-3脂肪乳的体内富集时间更短(术后第六天即检测到病患血清磷脂和红细胞细胞膜中的EPA和DHA显著升高)[35],激发免疫更强(第六天起外周血细胞释放的白三烯B5含量已增长约2倍)[36];
参考文献
1.Bone, R.C., C.J. Grodzin, and R.A. Balk, Sepsis: a new hypothesis for pathogenesis of the disease process. Chest, 1997. 112(1): p. 235-43.
2.Dobson, G.P., Addressing the Global Burden of Trauma in Major Surgery. Front Surg, 2015. 2: p. 43.
3.Binkowska, A.M., G. Michalak, and R. Slotwinski, Current views on the mechanisms of immune responses to trauma and infection. Cent Eur J Immunol, 2015. 40(2): p. 206-16.
4.Molfino, A., et al., Omega-3 Polyunsaturated Fatty Acids in Critical Illness: Anti-Inflammatory, Proresolving, or Both? Oxid Med Cell Longev, 2017. 2017: p. 5987082.
5.Alazawi, W., et al., Inflammatory and Immune Responses to Surgery and Their Clinical Impact. Ann Surg, 2016. 264(1): p. 73-80.
6.Hatakeyama, N. and N. Matsuda, Alert cell strategy: mechanisms of inflammatory response and organ protection. Curr Pharm Des, 2014. 20(36): p. 5766-78.
7.Matsuda, N., Alert cell strategy in SIRS-induced vasculitis: sepsis and endothelial cells. J Intensive Care, 2016. 4: p. 21.
8.Manson, J., C. Thiemermann, and K. Brohi, Trauma alarmins as activators of damage-induced inflammation. Br J Surg, 2012. 99 Suppl 1: p. 12-20.
9.Uddin, M. and B.D. Levy, Resolvins: natural agonists for resolution of pulmonary inflammation. Prog Lipid Res, 2011. 50(1): p. 75-88.
10.Barnig, C., N. Frossard, and B.D. Levy, Towards targeting resolution pathways of airway inflammation in asthma. Pharmacol Ther, 2018. 186: p. 98-113.
11.Walker, C.G., et al., The Pattern of Fatty Acids Displaced by EPA and DHA Following 12 Months Supplementation Varies between Blood Cell and Plasma Fractions. Nutrients, 2015. 7(8): p. 6281-93.
12.Mayer, K., et al., Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive Care Med, 2003. 29(9): p. 1472-81.
13.Novak, T.E., et al., NF-kappa B inhibition by omega -3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol, 2003. 284(1): p. L84-9.
14.Lo, C.J., et al., Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF kappa B activity. J Surg Res, 1999. 82(2): p. 216-21.
15.Calder, P.C., Omega-3 fatty acids and inflammatory processes. Nutrients, 2010. 2(3): p. 355-74.
16.de Gomez Dumm, I.N. and R.R. Brenner, Oxidative desaturation of alpha-linoleic, linoleic, and stearic acids by human liver microsomes. Lipids, 1975. 10(6): p. 315-7.
17.Hagve, T.A. and B.O. Christophersen, Effect of dietary fats on arachidonic acid and eicosapentaenoic acid biosynthesis and conversion to C22 fatty acids in isolated rat liver cells. Biochim Biophys Acta, 1984. 796(2): p. 205-17.
18.Hagve, T.A. and B.O. Christophersen, Evidence for peroxisomal retroconversion of adrenic acid (22:4(n-6)) and docosahexaenoic acids (22:6(n-3)) in isolated liver cells. Biochim Biophys Acta, 1986. 875(2): p. 165-73.
19.Lima-Garcia, J.F., et al., The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol, 2011. 164(2): p. 278-93.
20.Arita, M., et al., Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A, 2005. 102(21): p. 7671-6.
21.Aoki, H., et al., Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun, 2008. 367(2): p. 509-15.
22.Haworth, O., et al., Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol, 2008. 9(8): p. 873-9.
23.Serhan, C.N., et al., Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: a novel mechanism for NSAID and N-3 PUFA therapeutic actions. J Physiol Pharmacol, 2000. 51(4 Pt 1): p. 643-54.
24.Serhan, C.N., et al., Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med, 2002. 196(8): p. 1025-37.
25.Serhan, C.N., Pro-resolving lipid mediators are leads for resolution physiology. Nature, 2014. 510(7503): p. 92-101.
26.Serhan, C.N., N. Chiang, and T.E. Van Dyke, Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol, 2008. 8(5): p. 349-61.
27.Bastian, L., et al., [Clinical effects of supplemental enteral nutrition solution in severe polytrauma]. Unfallchirurg, 1998. 101(2): p. 105-14.
28.Al-Biltagi, M.A., et al., Beneficial Effects of Omega-3 Supplement to the Enteral Feeding in Children With Mild to Moderate Sepsis. J Intensive Care Med, 2017. 32(3): p. 212-217.
29.Galban, C., et al., An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit Care Med, 2000. 28(3): p. 643-8.
30.Kudsk, K.A., et al., A randomized trial of isonitrogenous enteral diets after severe trauma. An immune-enhancing diet reduces septic complications. Ann Surg, 1996. 224(4): p. 531-40; discussion 540-3.
31.Pontes-Arruda, A., A.M. Aragao, and J.D. Albuquerque, Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med, 2006. 34(9): p. 2325-33.
32.Atkinson, S., E. Sieffert, and D. Bihari, A prospective, randomized, double-blind, controlled clinical trial of enteral immunonutrition in the critically ill. Guy's Hospital Intensive Care Group. Crit Care Med, 1998. 26(7): p. 1164-72.
33.Farquharson, A.L., et al., Effect of dietary fish oil on atrial fibrillation after cardiac surgery. Am J Cardiol, 2011. 108(6): p. 851-6.
34.Kemen, M., et al., Early postoperative enteral nutrition with arginine-omega-3 fatty acids and ribonucleic acid-supplemented diet versus placebo in cancer patients: an immunologic evaluation of Impact. Crit Care Med, 1995. 23(4): p. 652-9.
35.Senkal, M., et al., Supplementation of omega-3 fatty acids in parenteral nutrition beneficially alters phospholipid fatty acid pattern. JPEN J Parenter Enteral Nutr, 2007. 31(1): p. 12-7.
36.Koller, M., et al., Impact of omega-3 fatty acid enriched TPN on leukotriene synthesis by leukocytes after major surgery. Clin Nutr, 2003. 22(1): p. 59-64.
37.Senkal, M., et al., Modulation of postoperative immune response by enteral nutrition with a diet enriched with arginine, RNA, and omega-3 fatty acids in patients with upper gastrointestinal cancer. Eur J Surg, 1995. 161(2): p. 115-22.
38.Iwase, H., et al., Nutritional Effect of Oral Supplement Enriched in omega-3 Fatty Acids, Arginine, RNA on Immune Response and Leukocyte-platelet Aggregate Formation in Patients Undergoing Cardiac Surgery. Nutr Metab Insights, 2014. 7: p. 39-46.
39.Gianotti, L., et al., Effect of route of delivery and formulation of postoperative nutritional support in patients undergoing major operations for malignant neoplasms. Arch Surg, 1997. 132(11): p. 1222-9; discussion 1229-30.
40.Schilling, J., et al., Clinical outcome and immunology of postoperative arginine, omega-3 fatty acids, and nucleotide-enriched enteral feeding: a randomized prospective comparison with standard enteral and low calorie/low fat i.v. solutions. Nutrition, 1996. 12(6): p. 423-9.
41.Daly, J.M., et al., Enteral nutrition with supplemental arginine, RNA, and omega-3 fatty acids in patients after operation: immunologic, metabolic, and clinical outcome. Surgery, 1992. 112(1): p. 56-67
公司邮箱:wlf-0471@163.com
销售热线:400-6758105

天猫店二维码

万利福公众号